Introduction:
Transformation of low grade follicular lymphoma (FL) to diffuse large B cell lymphoma (DLBCL) carries a poor prognosis. In retrospective studies, 5-year survival of transformed DLBCL treated with rituximab, cyclophosphamide, doxorubicin, vincristine and prednisone (R-CHOP) without autologous stem cell transplant is 40-60%, underscoring need to improve frontline treatment of transformed DLBCL beyond R-CHOP. The overall response rate (ORR) to lenalidomide used as a single agent for relapsed transformed non Hodgkin lymphoma was 45% with 21% complete response (CR) rate and a median duration of response of 12 months (Witzig et al, Ann Onc 2011). These data provided the rationale to include patients with transformed DLBCL (with historical and concurrent FL) in MC078E, a phase II clinical trial testing lenalidomide plus R-CHOP (R2CHOP) for patients with new and untreated de novo and transformed DLBCL (NCT00670358). Here we present analysis of the subset of transformed DLBCL patients.
Methods:
Adult patients with transformed DLBCL and either historical or concurrent FL, stage >=2, measurable disease by Positron Emission Tomography/computed tomography (PET/CT) and adequate organ function were included. Patients with Central Nervous System (CNS) involvement, significant comorbidities, active non-lymphomatous malignancy, life-threatening thromboembolism (TE) and contraindication to aspirin prophylaxis were excluded. Study participants received up to 6 cycles of rituximab (375 mg/m2), cyclophosphamide (750 mg/m2), doxorubicin (50 mg/m2), and vincristine (1.4 mg /m2) on day 1, prednisone (100 mg) on day 1-5, pegfilgrastim on day 2, and Lenalidomide 25 mg day 1-10 of 21 day cycle. Tumor lysis prophylaxis was per local practice; patients also received TE prophylaxis with aspirin. Primary outcome was event free survival (EFS) at 12 months, where an event was defined as death, progression or subsequent anti-lymphoma therapy. Secondary outcomes included ORR, CR, progression free survival (PFS), and overall survival (OS). Response was evaluated by PET/CT after cycle 2 and cycle 6 with revised response criteria (Cheson et al, 2007). Adverse events were recorded according to CTCAE version 3.0. The Kaplan-Meier method was used to estimate time to event endpoints.
Results:
Thirty-nine patients were accrued from August 5, 2013 to July 28, 2020 and 33 were eligible by central pathology review. Median age was 64 (range 24-80) years and 18 (54%) were >60 years old. Eighteen (54%) were male, and 32 (97%) had ECOG performance status <2. Twenty-three (70%) had historical FL and 10 (30%) had concurrent FL. Twenty-six (79%) had advanced stage (III-IV). Median number of extra nodal sites were 1 (0-4). Thirteen (39%) had high international prognostic index (IPI) (4-5). Thirty-two (97%) completed at least 2 cycles (30 completed all 6 cycles) and were evaluable for response.
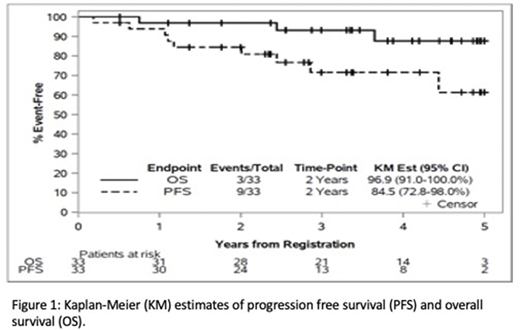
ORR was 97% (32/33), 29 (88%) had CR and 3 had PR. EFS at 12 months was 87.9% (95% CI: 71.8, 96.6). Two-year PFS and OS were 84.5% (95% CI: 72.8%-98%) and 96.9% (95% CI: 91-100%) (Figure 1). Twenty nine completed study protocol, 4 discontinued protocol early for disease progression (1), adverse event (AE) (1), refusal (1) and noncompliance (1). Thirty (91%) had hematologic AE of grade 3 or above, 27 (82%) had neutropenia, 16 (48%) had thrombocytopenia, and 7 (21%) had anemia. Sixteen (48%) had grade 3 or above non-hematologic AE. Eight (24%) had febrile neutropenia. There were 3 deaths on this study, 1 due to progressive DLBCL, 1 due to AML and 1 due to malignant melanoma.
Conclusion:
R2CHOP appears effective in transformed DLBCL with high response rates, event free, progression free and overall survival seen in current study. This study supports the inclusion of anthracycline naive patients with transformed DLBCL in future randomized studies of lenalidomide or other novel immunomodulatory (IMiD) analogues.
Wang:Novartis: Research Funding; Innocare: Research Funding; Incyte: Research Funding. Ansell:Bristol Myers Squibb: Research Funding; Takeda: Research Funding; AI Therapeutics: Research Funding; Regeneron: Research Funding; Trillium: Research Funding; Affimed: Research Funding; Seattle Genetics: Research Funding; ADC Therapeutics: Research Funding. Witzig:Spectrum: Consultancy; Immune Design: Research Funding; Karyopharm Therapeutics: Research Funding; Acerta: Research Funding; Incyte: Consultancy; AbbVie: Consultancy; MorphSys: Consultancy; Celgene: Consultancy, Research Funding. Nowakowski:Celgene/BMS: Consultancy, Research Funding; MorphoSys: Consultancy, Research Funding; Ryvu: Consultancy, Membership on an entity's Board of Directors or advisory committees; Kite: Consultancy; Kymera: Consultancy; Curis: Consultancy; Seattle Genetics: Consultancy; NanoString: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal